Downing portfolio company Adaptix receives 510(k) clearance from US FDA for its first medical imaging product
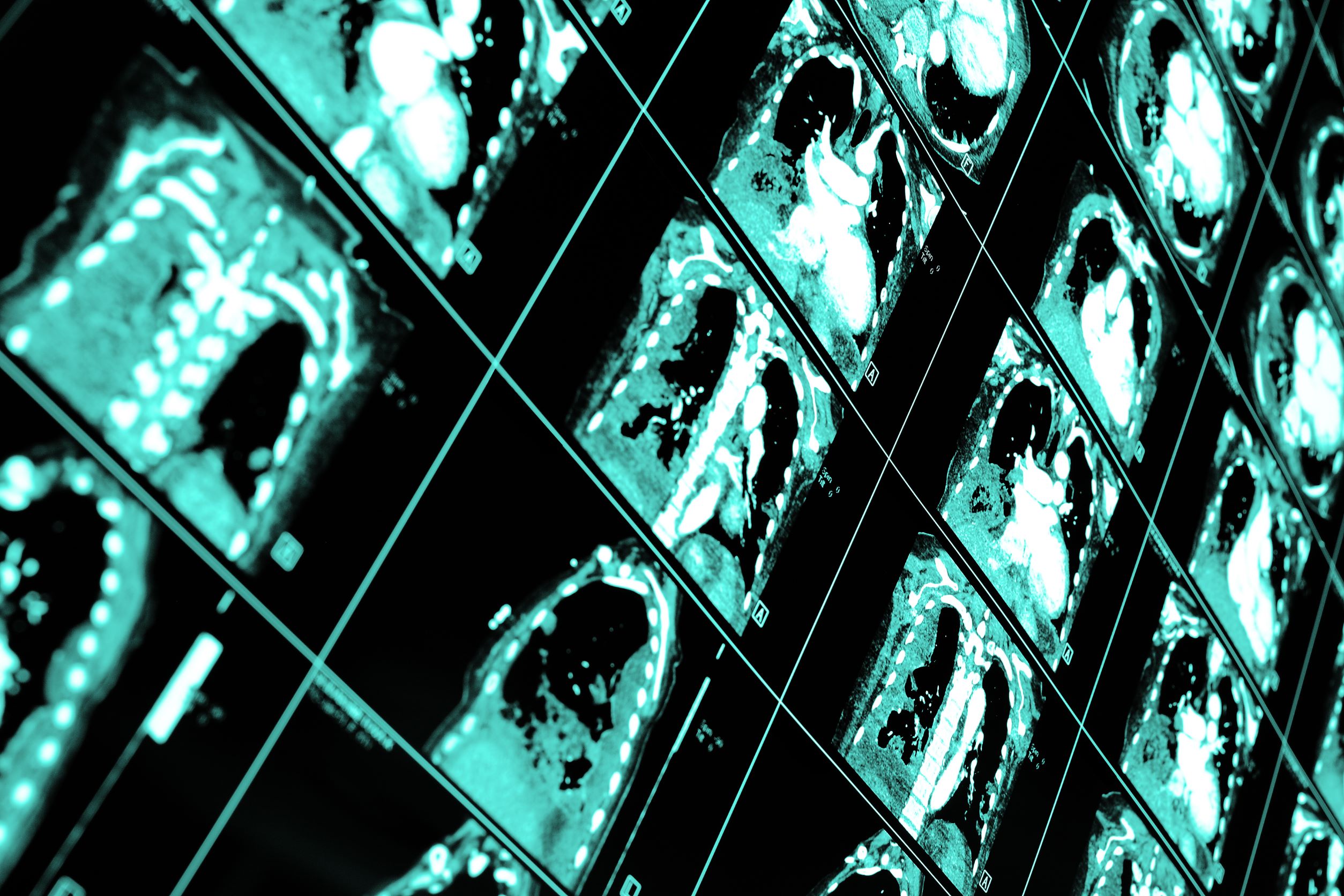
Adaptix, based at Oxford University Science Park, has now received 510(k) clearance from the US Food and Drug Administration, an important milestone that demonstrates that Adaptix’s device is safe, effective and marketable in the US.
Adaptix is transforming radiology through the development of innovative 3D imaging technologies. The first Adaptix medical product is a Digital Tomosynthesis Orthopedic imaging system. It is a portable, low-dose imaging system capable of delivering fast, lower-cost X-ray imaging at the point of patient care. It was developed specifically to offer 3D X-ray imaging of hands, elbows and feet at a fraction of the radiation dose and per-study price of traditional CT scanning systems.
Now that Adaptix has received 510(k) clearance, its technology can be marketed in the world’s largest healthcare market.
Read more about this news here
Adaptix, based at Oxford University Science Park, has now received 510(k) clearance from the US Food and Drug Administration, an important milestone that demonstrates that Adaptix’s device is safe, effective and marketable in the US.
Adaptix is transforming radiology through the development of innovative 3D imaging technologies. The first Adaptix medical product is a Digital Tomosynthesis Orthopedic imaging system. It is a portable, low-dose imaging system capable of delivering fast, lower-cost X-ray imaging at the point of patient care. It was developed specifically to offer 3D X-ray imaging of hands, elbows and feet at a fraction of the radiation dose and per-study price of traditional CT scanning systems.
Now that Adaptix has received 510(k) clearance, its technology can be marketed in the world’s largest healthcare market.
Read more about this news here
Adaptix, based at Oxford University Science Park, has now received 510(k) clearance from the US Food and Drug Administration, an important milestone that demonstrates that Adaptix’s device is safe, effective and marketable in the US.
Adaptix is transforming radiology through the development of innovative 3D imaging technologies. The first Adaptix medical product is a Digital Tomosynthesis Orthopedic imaging system. It is a portable, low-dose imaging system capable of delivering fast, lower-cost X-ray imaging at the point of patient care. It was developed specifically to offer 3D X-ray imaging of hands, elbows and feet at a fraction of the radiation dose and per-study price of traditional CT scanning systems.
Now that Adaptix has received 510(k) clearance, its technology can be marketed in the world’s largest healthcare market.
Read more about this news here
Adaptix, based at Oxford University Science Park, has now received 510(k) clearance from the US Food and Drug Administration, an important milestone that demonstrates that Adaptix’s device is safe, effective and marketable in the US.
Adaptix is transforming radiology through the development of innovative 3D imaging technologies. The first Adaptix medical product is a Digital Tomosynthesis Orthopedic imaging system. It is a portable, low-dose imaging system capable of delivering fast, lower-cost X-ray imaging at the point of patient care. It was developed specifically to offer 3D X-ray imaging of hands, elbows and feet at a fraction of the radiation dose and per-study price of traditional CT scanning systems.
Now that Adaptix has received 510(k) clearance, its technology can be marketed in the world’s largest healthcare market.
Read more about this news here

Please fill out the form to download the full report
Downing LLP does not provide advice or make personal recommendations and investors are strongly urged to seek independent advice before investing. Investments offered on this website carry a higher risk than many other types of investment and prospective investors should be aware that capital is at risk and the value of their investment may go down as well as up. Any investment should only be made on the basis of the relevant product literature and your attention is drawn to the risk, fees and taxation factors contained therein. Tax treatment depends on individual circumstances of each investor and may be subject to change in the future. Past performance is not a reliable indicator of future performance. Downing LLP is authorised and regulated by the Financial Conduct Authority (Firm Reference Number 545025). Registered in England No. OC341575. Registered Office: Downing, 10 Lower Thames Street, London, EC3R 6AF.









